Persistence of Touch DNA for Analysis
NIJ-funded research reveals how long DNA is detectable on various surfaces under different conditions.
National Institute of Justice
Since the first use of DNA evidence in a criminal case in 19861, forensic scientists have considered biological material (such as hair, skin, and bodily fluids) to be relatively reliable physical evidence.
While early technology required a substantial amount of biological material to extract enough DNA to build an individual profile for analysis, researchers have since discovered that they can obtain reliable DNA from more than just bloodstains or visible fluids; they can also obtain it from “touch DNA” that is left behind on surfaces or objects such as doorknobs, window latches, or steering wheels. Although touch DNA can be essential for forensic casework, it also comes with its share of issues, including those related to:
- Low quantity of useable DNA.
- High variability in the amount of DNA left by touch; that is, high variability in the amount that one person leaves, and high variability in the amount left from person to person.
- DNA degradation, including the many factors that can cause DNA to break down over time.
The results from rigorous analysis of these complicated factors have important implications for how touch DNA is collected, analyzed, and interpreted.
In 2018, the Forensic Technology Working Group at NIJ called for “comprehensive, systematic, well controlled studies that provide foundational knowledge and practical data about ‘touch evidence’ persistence in the real world.” That same year, Dr. Meghan Ramsey’s group at the Massachusetts Institute of Technology (MIT) Lincoln Laboratory began quantifying how long touch DNA would persist on certain surfaces under specific conditions. Building on that knowledge, and in collaboration with Dr. Ramsey, scientists at South Dakota State University created predictive models of how DNA degrades on different surfaces under a range of environmental conditions.
Testing for Persistence: Dry and Hot Degrades DNA
The researchers addressed two central questions:
- How do surface type, environmental condition, and exposure time affect the stability of touch DNA evidence?
- Does the stability of touch DNA samples differ from control DNA samples?
To address these questions, scientists deposited control DNA and touch DNA samples2 onto steel bolts and cotton fabric swatches. Then, they examined the DNA residue over time, across varying temperature and humidity combinations, and under UV light exposure (Figure 1).3, 4
Researchers measured:
- The amount of DNA.
- The quality of DNA using a degradation index.
The ability to obtain a DNA profile using short tandem repeats (or STRs), commonly used in forensic genetic analysis.
Results indicated:
- As expected, the amount of DNA that persisted on the steel and cotton decreased over time (Figure 2).
- Of the varying environmental factors, UV light had the biggest effect on DNA degradation on both materials.
- The amount of DNA left by touch varied more than in the control samples.
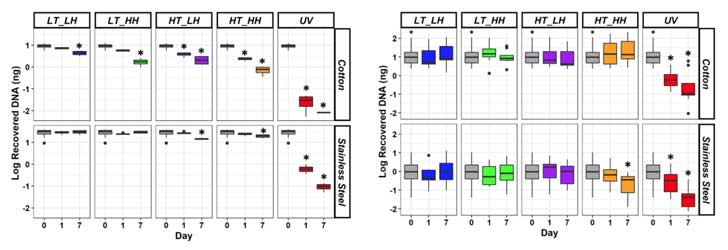
To predict the amount of DNA degradation over time, Dr. Ramsey worked with her collaborators to fit the DNA degradation data (based on temperature and humidity exposure) to a linear, mixed effects model.5 In doing so, they found:
- The mixed effect model was able to predict the extent of DNA degradation.
- DNA degraded more in high temperature and low humidity.
- Conversely, DNA samples were more stable at low temperatures.
To further examine DNA degradation, Dr. Ramsey and colleagues compared the completeness — whether the DNA profiles could be submitted to a database for a potential match — of two DNA profiles: environmentally exposed touch DNA recovered from steel bolts and unexposed reference sample DNA from cheek cells (Figure 3).
Notably:
- For all samples exposed to UV light, the researchers could not determine the DNA profiles. In other words, DNA samples exposed to UV light were too highly degraded to be useful in a forensic analysis.
- Under most conditions, all samples deposited on steel were highly stable. A noteworthy exception occurred under UV light exposure.
- The completeness of the DNA profiles determined from the touch DNA samples compared to cheek swab samples varied substantially across all environmental conditions.
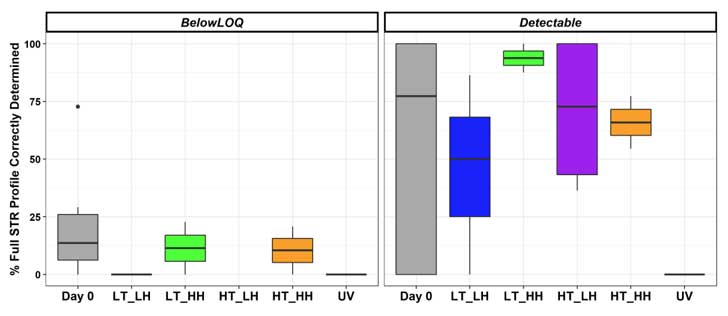
Figure 3: Percentage of complete STR profiles determined for touch DNA samples exposed to different environmental conditions. Results are shown separately for samples with DNA quantities below the limit of quantitation (LOQ) for the assay and for those that are above the LOQ (detectable). Touch DNA samples were one exposed to the temperature/humidity combinations for seven days. The UV light exposure was day. 6
Is There Not Enough DNA or Is It Degradation?
Throughout the course of this research, low and variable quantities of touch DNA collected remained a challenge; the low quantities of the initial touch DNA that scientists could recover made it difficult for researchers to evaluate the level of DNA degradation properly. Future work aims to increase the initial amount of touch DNA collected to record its degradation more accurately over time.
Still, those in forensics and law enforcement can glean valuable information from this ongoing research regarding the persistence of DNA in certain environmental conditions. For instance, investigators are more likely to recover useable DNA in cool and dry indoor environments than hot and humid outside conditions. Moreover, they may have better success obtaining DNA from stainless steel objects than fabric.
“The results from the study have generated a number of recommendations for best practices that the forensic science community can use to interpret and evaluate touch DNA evidence in a laboratory setting,” notes Physical Scientist Dr. Tracey Johnson of the National Institute of Justice.
Collectively, these studies provide the most comprehensive information to date on the persistence of touch DNA evidence.
About This Article
The work described in this article was supported by NIJ grant number 2018-DU-BX-0192 awarded to MIT Lincoln Laboratory.
This article is based on the grantee report, “Persistence of Touch DNA for Forensic Analysis” (pdf, 24 pages), by Meghan Ramsey.
Notes
- Z. Wong et al., “Characterization of a panel of highly variable minisatellites cloned from human DNA,” Annals of Human Genetics 51 (1987): 269–288, https://doi.org/10.1111/j.1469-1809.1987.tb01062.x.
- The control DNA came from seven donors and comprised 220 samples, and the touch DNA came from eight donors and comprised 408 samples. Exposure time was 14 days for control DNA and seven days for touch DNA.
- UV light was set up to mimic natural UV light irradiance in the northern and southern hemispheres. UVA and UVB light (ranging from 280-400 nm) was used, and this is considered a moderate UV light exposure.
- The research was focused on DNA persistence and does not address the other issues DNA studies often examine such as transfer, prevalence, and recovery.
- They did not include data on UV radiation and degradation in the modeling because UV radiation degrades DNA so much that DNA was below the level of quantification.
- The following numbers of samples were included in the analysis. For below LOQ samples: day zero (n=7), LT-LH (n=1), LT-HH (n=2), HT-LH (n=0), HT-HH (n=2), UV (n=2). For detectable samples: day zero (n=13), LT-LH (n=3), LT-LH (n=2), HT-LH (n=4), HT-HH (n=2), UV (n=1).
Cite this Article
National Institute of Justice, "Persistence of Touch DNA for Analysis," June 5, 2023, nij.ojp.gov: https://nij.ojp.gov/topics/articles/persistence-touch-dna-analysis
Read the report, “Persistence of Touch DNA for Forensic Analysis” (pdf, 24 pages), by Meghan Ramsey.

