Organic Gunshot Residue Analysis for Potential Shooter Determination
National Institute of Justice
Office of Investigative and Forensic Sciences

Executive Summary
Through the National Institute of Justice (NIJ) Forensic Technology Center of Excellence (FTCoE), West Virginia University (WVU) evaluated emerging approaches for the detection of gunshot residue (GSR) based on organic materials in the residue. One instrument was evaluated for screening for elemental constituents. This study examined x-ray fluorescence (XRF, a portable instrument), ion mobility spectrometry (IMS), and mass spectrometry. In summary, the study found the following:
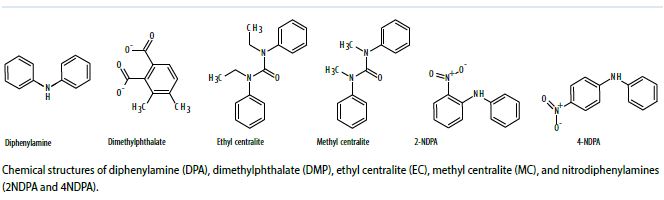
- The typical components of organic GSR (OGSR) include diphenylamine (DPA), ethyl centralite (EC), dimethyl phthalate (DMP), 2-nitrodiphenylamine (2NDPA), and 4-nitrodiphenylamine (4NDPA).
- OGSR residues should be detectable on skin for many hours after a firing event of as few as one or two gunshots.
- OGSR residues are not lost to secondary transfer. Residues remain detectable for 12 to 24 hours, with the mechanisms of loss being evaporation and skin permeation. The degree of loss varies from compound to compound.
- Existing pharmaceutical models can be used to estimate loss from the skin due to evaporation and permeation as a function of the compound and time elapsed.
- Hand swab samples are stable for approximately 2 weeks when stored at -20° C. After this period, significant degradation of some of the more volatile compounds is evident. Thus, OGSR samples have a holding time limit.
- The ability to detect specific OGSR compounds collected from hands at some time post-firing depends on the time elapsed, evaporative loss, loss to skin permeation, sampling efficiency, storage conditions, sample preparation, and instrumental method.
- The variation of persistence among OGSR compounds may lead to a viable method to estimate the time since deposition (i.e., time interval between a firearm discharge and the sampling event).
- The performance of any instrument as a screening device is generally dictated by the hand swabbing technique, not any inherent limitations with the instruments. Both collection of GSR from the subject and extraction into an instrument are important variables.
- It is recommended that standard reference samples (OGSR-impregnated swabs) be prepared for proficiency testing and other quality assurance/quality control purposes.
- XRF was examined in this study as a technique to screen hand swabs for the presence of metals associated with GSR (lead, barium, antimony). Because XRF is a non-destructive analytical method, it may be combined with other methods in the field or laboratory in sequence to produce effective screening. Lead is the most useful target element for XRF. Barium screening was found to be ineffective, and antimony appeared in only a very few positive samples. GSR detections based only on lead may be subject to false positives.
- Although it is possible to perform IMS analyses in the research laboratory, significant work remains before IMS can be employed reliably in the field for OGSR screening purposes. The key development needed is a large population study and generalization of pattern-matching algorithms for differentiating shooters from non-shooters, along with an associated probability.
- Differential mobility spectrometry (DMS) is a promising alternative to IMS for OGSR detection, though additional work is required before a full validation study can be done.
See also the instructional video "Crime Scene Reconstruction"
Gunshot Residue (GSR)
Firearms produce a range of residues upon discharge, including chemicals from the primer, explosive, oxidizers, reducing agents, sensitizers, fuels, and binders (Romolo & Margot, 2001). Collectively, these materials are often referred to as gunshot residue (GSR). In current forensic practice, GSR refers specifically to particulate residues that are formed from compounds found in the primer, including metal oxides. The term “primer discharge residue” is sometimes used to make this distinction clear. The propellant is ignited by the primer. The propellant burns and generates the gas that forces the bullet out; it is the source of the organic gunshot residue (OGSR). Both GSR and OGSR are deposited on the hands of the shooter. Particles can also be transferred to clothing or others nearby—a process referred to as secondary transfer. Because of the possibility of secondary transfer and environmental interferences, it can be challenging to interpret analytical results, be they positive or negative. The analysis of OGSR could add to the evidentiary value of GSR evidence by providing additional information from a source of evidence not prone to secondary transfer.

Current laboratory-based forensic analytical methods that target residue from firearms discharge focus almost exclusively on primer residues. Typical primer chemical composition includes lead styphnate (initiator), antimony sulfide (fuel), and barium nitrate (oxidizer), which combine in a violent chemical reaction that ignites the gunpowder in a cartridge. The primer materials vaporize and re-condense to form tiny particulates that contain lead, antimony, and barium, and these particulates can be collected using metal stubs coated with carbon tape.
The presence of GSR on a stub is determined using a combination of scanning electron microscopy (SEM) coupled to x-ray spectroscopy, usually energy dispersive x-ray spectroscopy (EDS). The SEM is used to locate particulates that are within the right size range and that have a smooth, rounded morphology. The chemical composition is evaluated using EDS, which identifies particles containing the three metals of interest. This method is described in the American Society for Testing and Materials (ASTM) E1588 Standard Guide for Gunshot Residue Analysis by Scanning Electron Microscopy/ Energy Dispersive X-ray Spectrometry. The combination of SEM/EDS for characterization of GSR has been accepted for many years in forensic science.
Organic Gunshot Residue (OGSR)
Because of the limitations of traditional GSR analysis, researchers have examined the possibility that other residues and analytical methods may increase the reliability and utility of results and permit field testing to aid investigations in real time. GSR contains a wide range of organic compounds such as diphenylamine (DPA, a stabilizer). Work in this study focused on six organic compounds: DPA, dimethylphthalate (DMP), ethyl centralite (EC), methyl centralite (MC), and nitrodiphenylamines (2NDPA and 4NDPA). Overall, compounds such as these make up less than 2% of most propellant formulations, but that amount is sufficient to be detectable after a firing event.
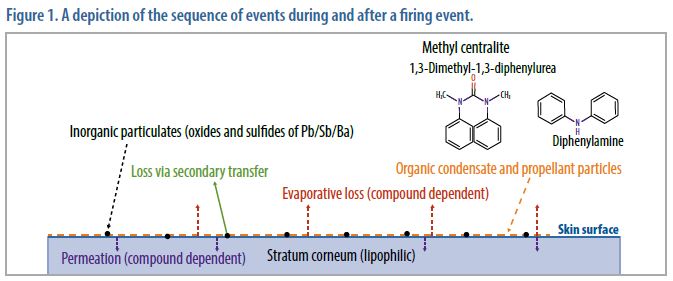
The way in which OGSR is deposited on the skin differs from the way inorganic, particulate GSR is deposited. Similar to inorganic GSR deposition, particles containing organics—such as unburnt or partially burnt propellant—can be physically deposited on the skin, and when deposited as solids, are subject to secondary transfer. However, organic compounds can also be volatilized during a firing event and may re-condense on the skin surface. These volatilized compounds adhere to the skin surface through lipophilic interactions and are not prone to secondary transfer. Although the compounds are still subject to loss, the mechanism of loss is a combination of evaporation and permeation into the skin. As depicted in Figure 1, a mixture of particulates and condensed organic compounds are deposited on the skin surface (the stratum corneum). This loss takes place over several hours and varies by compound. Also, OGSR compounds are uncommon outside of their use as explosive stablizers, so it is likely that OGSR would be less prone to environmental interferences than traditional GSR analysis.
Organic GSR adheres to the surface of skin in a manner that is different from traditional GSR particles. GSR is dispersed and deposited as particles, while organic GSR is dispersed and deposited as a vapor. These compounds are best recovered using a solvent-based wiping process. In this study, WVU identified muslin and Nomex® as sampling media that efficiently collect both types of GSR residue.
Organic GSR Sampling
Hand swabbing is used to collect samples for OGSR. Unlike traditional GSR, components of OGSR can adhere to and penetrate into the skin; therefore, it is important to optimize the sampling media for collecting OGSR. Optimal sampling methods for OGSR require that absorbent swab materials be used to maximize collection efficiency.
The criteria used for OGSR sampling media is that it be:
- wettable using a benign solvent such as isopropanol or ethanol,
- compatible with field instrumentation for screening (in this study’s evaluation: x-ray fluorescence [XRF] and ion mobility spectrometry [IMS]),
- extractable for use with gas chromatography/mass spectrometry (GC/MS) or liquid chromatography/mass spectrometry (LC/MS) instrumentation (depending on the system used for laboratory analysis),
- compatible with SEM/EDS systems (for traditional GSR analysis),
- commercially available at reasonable cost,
- contaminant-free for the compounds of interest,
- vendor-neutral to the extent possible.
There are two steps that affect the overall efficiency of the procedure: sample collection efficiency from the skin, and desorption/extraction of the swab for instrumental analysis.
These parameters are dependent on the time of sampling, given that OGSR begins to evaporate and/or absorb into the skin as soon as deposition occurs. The deposition event itself depends on many uncontrollable variables, including the orientation of the weapon; the type of weapon; the ammunition used; the number of shots fired; and environmental conditions.
This study showed that two sampling media met the above criteria: muslin and a Nomex® blend. These materials can be cut to order and are adaptable to a range of instrumentation. The swabbing procedure was conducted such that residues were collected on a relatively small surface area, but an area that was sufficiently large to allow for more than one independent analysis.
Once the sampling method was finalized, the stability of hand swab samples was characterized as a function of storage conditions. For each ion mobility spectrometer, 12 samples were prepared on swabs using four compounds: DPA, DMP, MC, and EC. Specifically, 1.0 microliter of each of the respective stock solutions was spiked separately on the swabs on top of one another. The resulting deposition approximated the range of expected concentrations on a hand swab of a shooter assuming 1 to 2 shots and collection of the sample within an hour of firing.
The stability of the collected swabs was evaluated as a function of two storage locations: 1) room temperature and under indoor lighting, and 2) in a dark freezer held at -20° C. The samples were analyzed as a function of storage time in both conditions.
The samples degraded significantly faster under the room temperature conditions. As expected, the most volatile compounds were lost rapidly, particularly DMP, and after a few days at room temperature, OGSR compounds could no longer be reliably detected. Although more study is needed to refine the holding time, it is clear that hand swabs of OGSR samples require cold, dark storage conditions and should be analyzed within 2 weeks of sample collection.
Population Study
Very little research has been done to examine the incidence of OGSR in the general population. This study did not seek to remedy that research gap, but did collect background and OGSR data from individuals in the general public and law enforcement. These collections enable the examination of GSR residue from a wide range of individuals to provide foundational reference data.
In Phase 1 of the project, 76 persons were sampled. In Phase 2, more than 200 people volunteered for sampling and completed a questionnaire regarding recent handling of firearms. All hand swab samples were collected under an approved Institutional Review Board (IRB) protocol.
Phase 1
The results of Phase 1 are outlined below. The study used GSR collected from the following individuals:
- Known positives: 76
- Blanks: >200 (instrument, swab, hand)
- False negative: Shooter washing hands, 25
- False negative: Shooter using sanitizer, 25
Significant attention was directed towards collecting potential false positive and false negative samples given the issues with GSR analysis of particulates and the potential for secondary transfer.
Phase 2
Data were collected from 191 individuals from the general public and law enforcement. Of these, 51% (97 individuals) reported that they shoot firearms as part of their profession, for hunting, or as a hobby. Approximately 23% had discharged a firearm in the past month. Of these, 41% reported that they typically discharge 11 to 50 rounds of ammunition in a typical shooting session. Many reported frequent physical contact with ammunition and firearms, regardless of whether they had fired a weapon. Although these data are not definitive, they suggest that positive GSR tests may not be indicative of criminal activity for some individuals based on their recent history. Data analysis of these swabs is on-going.
Persistence of OGSR on the Hand
OGSR components are lipophilic in nature. In other words, they tend to combine with or dissolve in fats, so they adhere to skin. Consequently, organic residues are expected to be less prone to secondary transfer. This was demonstrated in previous work by WVU; however, persistence was limited to periods ranging from 3 to 24 hours. Absent the deliberate removal by hand washing, only two loss mechanisms are feasible: evaporative loss and dermal absorption. These mechanisms must be characterized and understood before this type of physical evidence can be exploited. The key factors for such utilization will be the efficacy of sample collection from the skin; the efficiency of the sample extraction and preparation; and the instrumental limits of detection and quantitation. Underlying all of these issues is the elapsed time since deposition, which will determine the degree of evaporative and absorptive loss of each target compound.

After a 24-hour cycle on a skin surrogate (polydimethylsiloxane, or PDMS), detectable amounts of three compounds associated with OGSR (i.e., DPA, EC, and 4NDPA) penetrated the skin surrogate and were found in the receptor fluid. This is the same procedure used in pharmacological and toxicological studies to model skin permeation. DMP and 2NDPA were not found in the receptor fluid and thus would not be expected to permeate into the skin in significant quantities. This may be due to the smaller amount of the latter two compounds in the initial deposition, or to other factors such as surface loss rates (evaporation or absorption), which dictate how long OGSR compounds will remain at recoverable concentrations on the hands of shooters. The amounts that were initially placed on the PDMS substrates correlated to amounts expected to be deposited during an authentic firing event of 1 to 2 gunshots with a 9 mm pistol; this amount was determined via field experiments of actual firings (Bell, 2014).
Evaluation of Utility of OGSR
The utility of OGSR as physical evidence as a means of differentiating a shooter from a nonshooter depends on several factors. These factors include the time since deposition, efficacy of the sample collection process (hand wipes, hand swabs), sample preparation efficiency, and instrumental figures of merit (limit of detection and limit of quantitation). While deposition events (see Figure 1, above) that follow a firearm discharge are heterogeneous by nature and influenced by many variables, these variables do not preclude the use of OGSR as a form of physical evidence, particularly where the forensic question relates to the likelihood that the person recently did (or did not) fire a weapon.
A significant advantage of OGSR evidence is that losses due to secondary transfer are negligible, but the factors that contribute to this persistence (primarily lipophilicity) mean that the compounds are lost to evaporation and absorption, the rates and amounts of which are compound-dependent. This knowledge, derived experimentally and by modeling, provides a dimension of value missing from particulate GSR. Thus, it may be possible to derive an estimate of time since a firing event from the relative concentration of OGSR compounds recovered on the hands.
This study showed that skin swabbing methods are effective for collecting OGSR. As time elapses, the degree of evaporation and absorption dictate what compounds can be recovered and in what amounts. This work also shows that the ideal target compounds for OGSR characterization recovered from skin are those with low evaporative loss coupled with slow permeation characteristics such as EC, 2NDPA, and 4NDPA. Finding DPA or DMP on the hands would indicate that the firing event occurred within a few hours of collection, while absence of these compounds suggests that more time has elapsed. Given the right combination of sampling method and analytical procedure, it may be possible to detect OGSR for nearly 24 hours after a firing event. Thus, both traditional GSR particulates and OGSR compounds have a window of detection of ~12-24 hours. The ability to target multiple compounds via OGSR is also an advantage over traditional GSR methodology. Ideally, the two methods could be combined to provide a robust method for determining the likelihood that a person has fired a weapon within 12-24 hours. The specific methods and sequence of analysis requires further study and refinement.
Novel GSR Detection Methods
In prior studies at WVU and elsewhere, several detection methodologies for inorganic and organic GSR have been examined for use in the forensic laboratory, including GC/MS, LC/MS, and Raman spectroscopy (Dalby, Butler, & Birkett, 2010). In this study, IMS was the primary method evaluated for OGSR detection. In addition, a preliminary study of differential mobility spectrometry—a variation on IMS—was also conducted. IMS and XRF would be considered as presumptive testing in this context, not definitive or confirmatory.
XRF was examined as a possible complement to IMS for field studies because XRF can detect the elements associate with GSR (lead, antimony, and barium). XRF is a form of atomic spectroscopy that characterizes metals based on displacement of inner shell electrons. Because inner shell electrons (not involved in bonding) are targeted, the chemical form of the metal does not matter, e.g., the x-ray absorbance of lead is the same as that of lead oxide. Generically, x-ray instrumentation consists of an x-ray source that can be scanned to produce a range of excitation wavelengths and a photon detector. Older and larger bench-top systems utilize liquid nitrogen–cooled detectors to minimize electronic noise. The XRF instrument evaluated here used thermoelectric cooling (Peltier cooling).
XRF and IMS are complementary methods that detect different components of GSR. XRF detects traditional, inorganic primer residues, while IMS detects organic compounds from stabilizers. XRF is non-destructive, so it does not interfere with subsequent analyses of any type. When used in sequence, positive results from both XRF and IMS would afford greater confidence in a shooter/non-shooter determination. This does not eliminate the need for confirmation, but at least laboratories would have a rapid and relatively inexpensive method for screening samples prior to confirmation. Finding both OGSR and elemental components of inorganic GSR adds another dimension of information never before fully exploited in forensic settings.
IMS is a gas-phase ion-separation method that operates at atmospheric pressure. In all IMS instruments evaluated here, the sample is collected by swabbing, and a portion of the swab is placed in a thermal desorber associated with the instrument. Compounds volatilize and enter the ionization region of the instrument. In this region, thermal electrons from a radioactive source (63Ni β emitter) initiate a series of ionization steps that result in the formation of ion/molecule clusters. In the absence of sample (air only), reactant ions are typically H(H2O)n+ for the positive ion mode and O2- in the negative ion mode. In commercial instruments, other compounds may be used as the reactant ion, but the mechanism of formation is analogous. Ions collect in the ionization region and are introduced into the drift region by pulsing an electronic shutter. The ions travel under the influence of an electrical field gradient toward the detector. Opposing this motion is a flow of clean, dry drift gas (also air in these instruments) that collides with the ions. The ions are slowed in proportion to their size, with larger ions having longer drift times and smaller ions having faster drift times. Drift times are 10 to 20 minutes, and the output is a mobility spectrum that plots signal intensity as a function of drift time. The mobility spectrum is a plot of intensity at the detector plate as a function of drift time. In this study, it was found that the positive ion mode was much more useful for OGSR than the negative mode, so only the positive ion mode is discussed.

An analytical signal is generated in most cases via charge (proton) transfer. When samples are introduced into the ionization region via the thermal desorber, they are ionized as well. If these ions have a greater proton affinity than the reactant ion—H(H2O)n+ or other—the analyte acquires the charge, enters the drift region, and moves under the influence of the electric field to the detector. The reactant ion loses the proton (and thus its charge) and becomes a neutral atom that will not move under the electric field influence.
However, because of differences of design, variation in atmospheric conditions, and variations in operational parameters, the drift time (td) of a given compound can change from day to day and will not be the same between instruments. To address this, the drift time can be converted to mobility (K) and then to a reduced mobility value, referred to as K0. However, in this study, it was the pattern of peaks that was proven to be of value, not the presence or absence of specific mobility peaks with specific K0 values.
When these instruments are used in the field or for screening purposes, detection of a substance is based on obtaining a response at a drift time (corrected for temperature and pressure) that corresponds to compounds of interest. For example, the IMS instruments evaluated are supplied with pre-programmed channels (i.e., drift time windows) defined for compounds such as drugs of abuse (e.g., heroin, cocaine) and explosives (e.g., TNT, RDX). Theoretically, detection of the organic components of GSR would be expected to be additive; it might be expected that channels could be defined for each OGSR compound, and detection of multiple peaks would be considered characteristic in a qualitative sense of OGSR. However, this was not the case here, and this result was not surprising for several reasons.
Because samples are introduced by thermal desorption, all compounds that are desorbed exist in the reaction region simultaneously. The IMS response is based on charge exchange from an existing pool of reactant ions, so there is a finite amount of charge (H+ ions in positive mode) available to be transferred. How these charges transfer depends on relative gas-phase basicity. Additionally, charge transfer at atmospheric pressure is a competitive and complex process in which ion/molecule clusters and dimers (homogenous and heterogeneous) can form. The products seen at the detector plate are not simple linear additions of the materials introduced into the ionization region, and as such, a peak-based approach was expected to be problematic and a pattern-based approach for data analysis will be required. Data analysis of the 190+ samples is ongoing using pattern-matching algorithms. This work seeks to establish the parameters for the application of IMS for OGSR screening in a manner similar to that used today for screening of drugs of abuse or explosives.
Instruments Evaluated
As shown in Table 1, several different instruments were evaluated for this study. Note that the XRF (Niton® XL3t) evaluation was conducted only in Phase 1; an instrument was not available for follow-up work in Phase 2. As a result, the XRF results do not reflect work with optimized sampling media and do not constitute a full validation study.
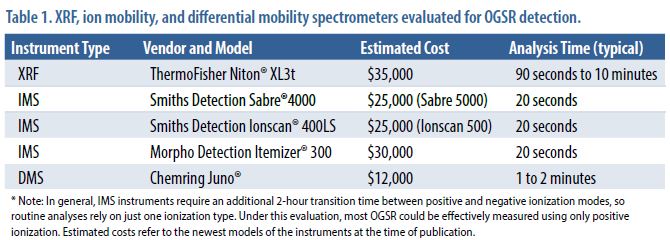
Two Smiths Detection instruments were evaluated: the Sabre®, which is a portable hand-held unit, and the Ionscan®, which is a bench instrument. Some differences between the instruments were found. Although the instruments’ drift tube designs and software are the same, there are minor differences in each instrument’s thermal desorption inlets.
For the Sabre, samples are inserted into a slot and the desorber actuates automatically, pressing against the swab and directing flow over the portion of the swab surface contacting the desorber. With the Ionscan, samples are cut from the swab and placed on a Teflon® membrane surface, which is then slid into position. The desorber presses the sample into the position. The only practical difference is that a smaller surface area is analyzed with the Ionscan compared to the Sabre. With both instruments, only part of the swab comes into contact with the desorber, preserving the remainder for confirmatory analysis as needed.
The Morpho Itemizer® instrument is based on a drift tube design capable of analyzing positive and negative mode ions simultaneously (or nearly so). This is accomplished using an ion-trap design for the reactant region. Ions are formed and trapped together, with different polarities being released by electronic pulses. This facilitates collection of data from both polarities from the same sample analysis. The Itemizer was tested using standards and hand swab samples. The detection limits were not low enough for this application, and the instrument does not readily provide raw data to the user, so additional data analysis was not possible.
X-Ray Fluorescence
The swabs used for the XRF studies in Phase 1 were the sampling media provided by the IMS instrument vendors and not the muslin and Nomex used in Phase 2. The background lead concentration on these swabs was 22.5 ppm +/- 0.98 ppm and 4.7 ppm +/- 4.1 ppm (95% confidence interval), respectively.
The swabs were rubbed over the entire surface of both hands of subjects who were known shooters and known non-shooters. XRF determined that the sampling produced heterogeneous concentrations of GSR across the swab, so multiple locations were sampled on a swab to develop a reliable measurement of mean concentration. Isopropanol-moistened swabs collected 3 to 4 times the lead and barium as non-moistened swabs. The presence of lead was found to be a reliable indicator that a swab was sampled from a shooter. Analysis of barium was complicated by high background detection levels of barium on the swabs, indicating sampling or interference issues. Antimony was seen only in a few cases at levels above the limit of detection, but all of these instances were associated with shooters. The infrequency of detection could be the result of ammunition composition rather than instrumental limitations. Given this correlation with shooters, it would be useful to continue to monitor all samples for lead and antimony.
The lead concentrations detected on the hands of known shooters who washed their hands after shooting and before sampling were indistinguishable from non-shooters; however, known shooters that used sanitizer instead of soap showed statistically significantly higher concentrations of lead.
In summary, XRF appears promising for use as a GSR screening tool and has the distinct advantage of being a completely non-destructive technique. However, a full validation study is needed using the optimized swab materials and protocols to judge the value of the XRF/IMS combination for rapid screening for inorganic GSR and organic GSR, respectively.
Ion Mobility Spectrometry
OGSR samples were examined using each of the IMS instruments. The primary target compound for optimization was DPA because it is the most common compound found in samples obtained from known shooters.
To set the initial instrument conditions for the three IMS instruments, a commercial OGSR solution was used. Depending on the instrument, 1–5 μL of solution was delivered to the swab surface, and the swab was allowed to air dry (~ 1 minute). Instrumental parameters were adjusted to maximize the response of the DPA, as determined by the amplitude of the associated mobility peak. In initial experiments to determine the viability of using the negative mode of the IMS, performance was optimized using a TNT solution. However, the negative mode proved of little value given the significantly higher backgrounds coupled with lack of consistent response for compounds expected to be in OGSR, such as TNT and nitroglycerin. This was observed for all three IMS instruments, and subsequent work for these three detector systems was conducted in the positive ion mode. Table 2 presents the detection thresholds for the two Smiths Detection instruments.
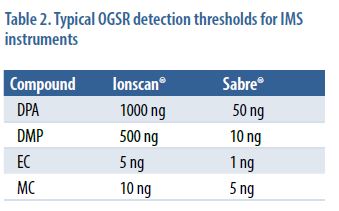
The amount of recoverable compounds deposited on skin during a firing event was determined to be in the range of 100–200 ng (0.1–0.2 μg), so these limits of detection are generally favorable and suggest that detection should be feasible as long as samples are stored under cold, dark conditions.
The Sabre values are lower, most likely due to the larger amount of the surface area of a swab being analyzed. In addition, the Sabre instrument was optimized for OGSR detection using DPA as a calibrant, while the Ionscan instrument was employed in its default mode.
IMS instruments cannot be used to quantify the amount of material in a particular sample because peak amplitudes will vary according to instrument conditions. In addition, drift times can vary if the instruments are not calibrated on a daily basis. The methodology demonstrated robustness across both instruments—the Smiths Detection Sabre and the Ionscan. Of the two, the Ionscan results showed fewer spectral artifacts, but both instruments performed acceptably. Because both are older (~ 9 years), heavily used instruments, this finding should not be generalized.
Differential Mobility Spectrometry
The Chemring Juno® instrument is a benchtop differential mobility ion mobility spectrometer (DMS) that is similar to IMS in that it measures the mobility of ions at atmospheric pressure. Ions are produced in the Juno using a Ni-63 source, which is the same method used in the IMS instruments. The key difference between IMS and DMS is in the way in which an electrical field is applied to the drift region. In IMS, the drift-tube region experiences a low-voltage field gradient that decreases steadily toward the detector plate. Unlike the constant voltage gradient applied to an IMS drift tube, a waveform of varying electric field strength is applied to two internal plates. Ions are separated based on their different responses to high and low fields between the plates. The mode of ion separation depends on mobility under both high and low field conditions or the differential mobility. One of the advantages of DMS is that positive and negative mode ions are characterized simultaneously. The Juno uses a heated cap inlet, which facilitates sample volatilization and introduction into the unit. Using heated air evaporation to introduce the sample is appealing in forensic applications because of the lack of sample depletion and the preservation of evidence on the swab for subsequent confirmatory analysis.
The Juno shows promise as a DMS instrument for use in OGSR, but more work is needed to validate the instrument and the method. The instrument collects data in the positive and negative mode simultaneously and produces a rich data set that includes patterns and extractable peaks. The ability to scan the rf (radio frequency) and compensation voltages introduces another level of discrimination beyond that of the IMS instruments. The key to progress here is validation, followed by chemometric analysis that would determine how best to extract information to provide a probabilistic assessment of the shooter/non-shooter question. It is also feasible to apply feature extraction and pattern-matching algorithms borrowed from tool mark analysis. Finally, the use of a heated-cap DMS permits multiple confirmatory tests on the same swab because the swab never contacts a desorber surface. The swab could be analyzed using SEM/EDS or mass spectrometry, although sample extraction methodologies must be further developed for that application.
Finally, attempts to extract the hand swabs for the purpose of confirming the detection of OGSR using LC/MS/MS and GC/MS had mixed success. The problems had to do with reproducibility and low recovery. The results suggest that other approaches to sample preparation and introduction should be pursued.
Conclusions
Traditional laboratory and field tests that rely on the detection of inorganic, primer-based residues may be limited in their effectiveness by environmental contamination and other factors. OGSR may improve the utility and reliability of determinations concerning whether a suspect has discharged a firearm. OGSR chemicals are less subject to environmental interferences, may provide a longer window of opportunity to detect firing events, and may provide a mechanism to estimate the time when a firing event occurred. Sampling must be carefully controlled to optimize collection efficiency and enable instrument detection. GC/MS and other laboratory-based methods may reliably be used to perform confirmatory and quantitative analyses. XRF and IMS may provide a robust set of field instruments that can detect both inorganic GSR and OGSR. Differential mobility spectrometry also holds promise for detection of OGSR.
Further test and evaluation activity is needed to optimize collection and analysis methods, provide a means to quantitatively characterize GSR data with probability estimates, evaluate instruments in realworld conditions, and develop recommendations for practitioners.
Acknowledgements
In addition to the Principal Investigator, Dr. Suzanne Bell, the following individuals at WVU contributed to this work:
- Brittany Yeager (senior doctoral student and principle laboratory scientist)
- Corey Nida (senior doctoral student)
- Nienke Roelfs (visiting scholar, forensic science student, Avans Technical Institute, the Netherlands)
- Christopher Freedman (undergraduate forensic chemistry student)
- Matthew Evans (undergraduate chemistry student [BS May 2013])
- Andrew Jefferson (former doctoral student)
- Lauren Seitzinger, James Stewart, Ryan Dross, and Katelyn Bustin (undergraduates)
All original photographs contributed by West Virginia University, C. Eugene Bennett Department of Chemistry.
References
- Bell, J. W. (2014). Skin permeation of organic gunshot residue: Implications for sampling and analysis. Analytical Chemistry, 6071-6079.
- Dalby, O., Butler, D., & Birkett, J. (2010). Analysis of gunshot residue and associated materials–A review. Journal of Forensic Sciences, 55 (4), 924-943.
- Romolo, F., & Margot, P. (2001). Identification of gunshot residue. Forensic Science International, 119 (2), 195-211.
Citation
Forensic Technology Center of Excellence (FTCoE). (2015). In-Brief: Organic Gunshot Residue Analysis for Potential Shooter Determination. U.S. Department of Justice, National Institute of Justice, Office of Investigative and Forensic Sciences.
Public Domain Notice
All material appearing in this publication is in the public domain and may be reproduced or copied without permission from the National Institute of Justice (NIJ). However, this publication may not be reproduced or distributed for a fee without the specific, written authorization of the NIJ, Office of Justice Programs, U.S. Department of Justice.
Forensic Technology Center of Excellence (FTCoE)
The FTCoE is a collaborative partnership of RTI International and its Forensic Science Education Programs Accreditation Commission (FEPAC)–accredited academic partners: Duquesne University, Virginia Commonwealth University, and the University of North Texas Health Science Center. In addition to supporting the National Institute of Justice’s (NIJ’s) research and development (R&D) programs, the FTCoE provides testing, evaluation, and technology assistance to forensic laboratories and practitioners in the criminal justice community. The NIJ funds the FTCoE to transition forensic science and technology to practice (Award Number 2011-DN-BX-K564).
The FTCoE is led by RTI, a global research institute dedicated to improving the human condition by turning knowledge into practice. With a staff of more than 3,700 providing research and technical services to governments and businesses in more than 75 countries, RTI brings a global perspective. The FTCoE builds on RTI’s expertise in forensic science, innovation, technology application, economics, DNA analytics, statistics, program evaluation, public health, and information science.

